Mechanism of Action – Nuedexta
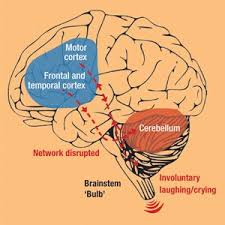
PseudoBulbar Affect(PBA) is a highly unfortunate condition that is known for causing sudden bursts of laughter or crying in patients. These outbursts come on quickly and frequently occur throughout the day. In some cases, there may be slight sadness or amusement before an outburst, but most often the outbursts are completely unprovoked.
More than 2 million people in America have been diagnosed with PBA. Most of these patients have either suffered a brain injury or another serious neurological disorder. Also, there are another 7 million patients in the country with symptoms that suggest they may be affected by PseudoBulbar Affect. Nearly half of the people who suffer from a traumatic brain injury begin to display symptoms of PBA.
It should not be confused with a condition like depression, which may result in symptoms that seem similar at first. PBA causes extremely exaggerated emotional expressions. These expressions are rarely an indicator of how the patient actually feels. PBA is a neurological condition that stems from damage to the nervous system. Depression, on the other hand, is a psychological condition that stems from problems with the patient’s mental or emotional state.
Modern PBA Treatment.
Today, PBA is often treated with a drug called Nuedexta. This medication is a potent drug combination that was developed by Avanir Pharmaceuticals, who is also the manufacture the drug. They began producing the drug in 2013, and it has since grown to become an influential and popular source of treatment for this neurological condition.
Avanir has not released the exact mechanism of action for Nuedexta, but most professionals believe it works by regulating certain parts of the nervous system. More specifically, it controls neurotransmissions that use the sigma-1 receptor as well as the NMDA receptor. Meanwhile, it has also been shown to slow down the brains breakdown of dextromethorphan.
Each capsule contains a combination of two drugs. You will find them in either 20/10mg combinations or 30/10mg combinations. One drug is dextromethorphan hydrobromide and the second drug is quinidine sulfate.
Nuedexta was only approved by the FDA after extensive clinical trials. The most recent trial involved giving some patients Nuedexta and some patients placebo. The study was conducted for a total of 12 weeks, and professionals measured the frequency and length of episodes of crying or laughter.
The study yielded very positive results for Nuedexta. The patients that used the drug suffered from significantly fewer episodes of crying or laughter compared to the patients who were given the placebo.
Another study was conducted before receiving approval from the CHMP. This was a somewhat similar study that focused on judging the safety and effectiveness of the drug in a total of 325 patients. It lasted for a total of 12 weeks and had nearly the same results.
The Bottom Line
Nuedexta is still a relatively new drug for treating PBA. The exact mechanism of action isn’t entirely known either. Still, it is impossible to argue with such positive results. It seems to be a very reliable treatment for patients suffering from this unfortunate condition.